Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the deadly disease known as coronavirus disease 2019 (COVID-19) that has reached pandemic proportions. Currently, there is no definitive treatment for COVID-19, although many vaccines have been developed. The World Health Organization has approved the safety and efficacy of the AstraZeneca/Oxford, Johnson and Johnson/Janssen (JnJ), Moderna, Pfizer/BioNTech, Sinopharm, and Sinovac vaccines so far. The approved formulations of AstraZeneca, JnJ, and Gam-COVID-vac (Sputnik V) contain DNA delivered within non-replicating recombinant adenovirus vector-based systems, while the Pfizer and Moderna vaccines utilize mRNA technology and lipid nanoparticle delivery systems. All of these vaccines encode production of the SARS-CoV-2 spike (S) protein, ultimately triggering immunity in the human body. COVID-19 causes several cardiovascular complications, such as arrhythmias, myocarditis, pericarditis, and venous thromboembolism. SARS-CoV-2 vaccines have been associated with rare, but sometimes fatal, cardiovascular side effects, which are the topics of this review. SARS-CoV-2 vaccines in general may cause thromboembolic events, such as cerebral vein thrombosis, and mRNA-based vaccines in particular may cause myocarditis/pericarditis, with the latter more likely to occur in younger adults after the second vaccination dose. Nevertheless, the advantages of these vaccines for ending the pandemic and/or decreasing the mortality rate outweigh any risk for the rare cardiovascular complications.
Similar content being viewed by others
COVID-19, which is caused by a new type of coronavirus named SARS-CoV-2 and become a worldwide pandemic, may cause cardiovascular complications. |
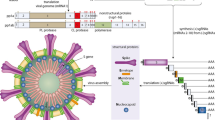
Most of the SARS-CoV-2 vaccines developed so far work by encoding production of the SARS-CoV-2 spike protein. |
SARS-CoV-2 vaccines in general may cause cardiovascular problems, although very infrequently. |
Rare, but sometimes fatal, cardiovascular events side effects may be associated with all types of SARS-CoV-2 vaccines. |
The advantages of the vaccines in terms of ending the pandemic and decreasing COVID-19 mortality outweigh the complications. |
Introduction
Coronavirus disease-2019 (COVID-19) is an infectious disease caused by a newly discovered coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Most of the individuals infected with COVID-19 will suffer from mild to moderate respiratory disease and recover without any additional therapy [1]. However, a number of risk factors increase the odds of developing severe illness, including old age and underlying medical problems, such as cardiovascular disease, diabetes mellitus, being overweight and obesity, chronic respiratory disease, and cancer [2, 3]. Although research projects have already being launched to find a definitive treatment for COVID-19, there is currently no confirmed curative treatment for the disease; accordingly, prevention is the fundamental principle for managing the COVID-19 pandemic. Effective vaccines are critical to ending the COVID-19 pandemic and lowering the mortality rate in infected individuals. The first mass vaccination program started in early December 2020, and as of 14 October 2021, 6,495,672,032 doses of vaccine have been administered globally [3]. By 3 June 2021, the World Health Organization (WHO) announced that the AstraZeneca/Oxford, Johnson and Johnson/Janssen (JnJ), Moderna, Pfizer/BioNTech, Sinopharm, and Sinovac vaccines are safe and effective [4]
Several types of vaccines for COVID-19 have been developed, some of which are approved and others are awaiting approval, or are under development, including: (1) RNA and DNA vaccines, a cutting-edge approach that uses genetically engineered RNA or DNA to generate a protein that safely prompts an immune response; (2) viral vector vaccines, which use a safe virus that cannot cause disease but serves as a platform to produce coronavirus proteins to generate an immune response; (3) inactivated or weakened virus vaccines, which use a form of the virus that has been inactivated or weakened in order not to cause disease but still generates an immune response; (4) protein-based vaccines that use harmless fragments of proteins or protein shells that mimic the COVID-19 virus to generate an immune response safely [4]. DNA delivered within a non-replicating recombinant adenovirus vector system is the platform used by the AstraZeneca, JnJ, and Sputnik V vaccines, while vaccines developed by Pfizer/BioNTech and Moderna use mRNA technology and lipid nanoparticle delivery systems [5,6,7,8]. Both the mRNA and adenovirus vector-based vaccines encode SARS-CoV-2 spike (S) protein production, the main target for neutralizing antibodies generated from natural infection and therapeutic monoclonal antibodies [9].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
COVID-19 and Cardiovascular Complications
COVID-19 may be associated with multiple serious cardiovascular complications, such as arrhythmias, myocarditis, and thromboembolism, either directly or indirectly [10]. SARS-CoV-2 causes disturbance to the renin–angiotensin system, increased angiotensin-II levels, and upregulation of pro-inflammatory cytokines that have destructive effects on the vascular endothelium and lead to a systemic inflammatory response syndrome that may provide a possible mechanism for multi-organ failure, usually involving the heart [11]. In addition, COVID-19 infection carries with it a substantial risk of developing blood clots [12]. In one study, the overall prevalence of pulmonary embolism in COVID-19 patients was 7.8%, deep vein thrombosis 11.2%, and stroke around 1.6% [12]. A summary of vaccine efficacy against the delta variant is given in Table 1.
Cardiovascular Complications of SARS-CoV-2 Vaccines
Severe side effects of vaccines may appear in some individuals vaccinated in mass vaccination programs. Also, given the short development time and the novelty of the technologies adopted, these vaccines may have serious side effects that will only be revealed after some time [13]. One potential mechanism is the free-floating spike proteins, synthesized by cells targeted by the vaccines, that circulate in the blood systematically interacting with angiotensin converting enzyme 2 (ACE2) receptors. These reactions may ultimately lead to platelet aggregation, thrombosis, and inflammation [14]. In this review, the potential cardiovascular complications of SARS-CoV-2 vaccines will be discussed.
Thrombembolic Events
Studies have suggested that SARS-CoV-2 vaccines that use an adenoviral vector platform may be related to the occurrence of thrombotic thrombocytopenia [15,16,17]. During early to mid-March 2021, several European countries stopped using the AstraZeneca vaccine because of spontaneous reports of thromboembolic events among vaccinated people [18, 19]. A study on recipients of the ChAdOx1 nCov-19 vaccine (AstraZeneca) reported that the standardized morbidity ratio for thromboembolic events was 1.97-fold higher among this study population than for the general population [20]. Some studies have reported that vaccination with the AstraZeneca vaccine can lead to immune thrombotic thrombocytopenia mediated by platelet-activating antibodies against PF4, a condition which clinically resembles severe heparin-induced thrombocytopenia [21, 22]. For example, in a study on ten patients who presented with one or more thrombotic events, including cerebral venous thrombosis, splanchnic-vein thrombosis, and pulmonary embolism, after AstraZeneca vaccination, the authors was concluded that vaccine-induced immune thrombotic thrombocytopenia occurred through the postvaccination formation of platelet-activating antibodies against PF4 [23]. In another study on 22 patients who received the AstraZeneca vaccine and subsequently presented with acute thrombocytopenia and thrombosis, including cerebral venous thrombosis, in the absence of previous prothrombotic medical conditions, the authors stated that pathogenic PF4-dependent syndrome could occur after the administration of the AstraZeneca vaccine [24]. Thereafter, similar thromboembolic events were reported in a study of patients who had received the JnJ vaccine (Ad26.COV2.S), a recombinant adenovirus serotype 26 vector encoding the SARS-CoV-2 spike glycoprotein. As of 14 April 2021, six cases of cerebral venous sinus thrombosis with thrombocytopenia, occurring 7–14 days after vaccination, were reported among more than 7.2 million people who had been vaccinated with JnJ globally [25]. Also, one patient developed deep vein thrombosis during a phase 3 trial on patients who received the Sputnik V vaccine [6]. There have also been cases of thrombosis reported in patients who had received the mRNA vaccines, with possible cases of central nervous system thrombosis among recipients of the Pfizer/BioNTech mRNA vaccine and Moderna mRNA vaccine [26].
Myocarditis and Pericarditis
Following COVID-19 vaccination (6 h to 4 days postvaccination), there have been case reports of myocarditis and pericarditis [27,28,29,30]. Two large retrospective studies were performed in Israel on the occurrence of myocarditis in persons who had received the Pfizer COVID-19 vaccine. Mevorach et al. [31] investigated over 5.1 million vaccine recipients 21 days after the first dose and 30 days after the second dose. They found 136 cases of what was most likely myocarditis, of which 95% were mild in symptoms and only one case was fatal. Also, the risk difference for developing myocarditis between the first and the second doses of the vaccine was 1.76 per 100,000 persons overall and 13.73 for male recipients aged 16- to 19-years. In the other Israel-based study, Witberg et al. [32] searched the records of over 2.5 million recipients of the Pfizer vaccine and found that the incident rate of the myocarditis was 2.3 per 100,000 persons, increasing to over ten cases per 100,000 persons among recipients aged 16- to 29 years. In addition, a search by Montgomery et al. [33] of US military personnel vaccinated with mRNA vaccines identified 23 cases of myocarditis within 4 days postvaccination. Another study reported that the risk of developing myocarditis is 18.28-fold higher in those who had been infected with SARS-CoV-2 than in those who are not infected, which is considerably high compared to the increased risk in vaccinated people (3.24-fold higher than in those who have not been vaccinated) [34]. Given that the symptoms appeared close to the vaccination time and ruling out other possible diagnoses, the authors of a number of studies have postulated that these complications were secondary to vaccination; however, the pathophysiology remains unknown [28,29,30]. Mechanisms of an unspecific inflammatory response secondary to vaccination and cross-reactivity of antibodies due to the molecular mimicry have been hypothesized. Nearly all reported cases of myocarditis occurred following vaccination with mRNA-based vaccines (Pfizer and Moderna). Watad et al. reported two cases of pericarditis after vaccination with mRNA-based vaccines [30], with the patients responding to anti-inflammatory and corticosteroid drugs. Diaz et al. [35] investigated more than 2 million vaccinated individuals and found 37 cases of pericarditis, with the median onset of 20 days for symptoms after vaccination; there was no mortality. The mean monthly number of patients with pericarditis in the pre-vaccination period (January 2019–2021) was significantly lower than that in the vaccination period between February and May 2021 (49.1 vs. 78.8). The risk ratio of developing pericarditis after vaccination with the Pfizer vaccine was reported to be 1.27 by Barda et al. [34], in comparison to 5.39 for those who were infected with SARS-CoV-2, as compared to healthy individuals. Up to 13 October 2021, the U.S. Center for Disease Control (CDC) [36] announced that myocarditis/pericarditis was a rare side effect that was more likely to happen in male adolescents and younger adults after the second dose of vaccination rather than the first dose and within several days after vaccination with mRNA-based vaccines. Furthermore, younger vaccine recipients must be more closely evaluated if they present with acute chest pain, palpitations, and shortness of breath for myocarditis/pericarditis following vaccination; it should be remembered that the acute coronary syndrome is less likely in this population [36]. Of note, other possible diagnoses based on the patient’s history should be considered [36, 37].
Arrhythmia
A case of paroxysmal ventricular arrhythmia was reported in the Pfizer vaccine (BNT162b2) clinical trial, but no causal relationship was found [8]. Of note, the occurrence of ventricular arrhythmias has been associated with cardiac inflammation [38]. García et al. reported three cases of tachycardia following Pfizer vaccination; all three patients had a previous history of infection with SARS-CoV-2 [39]. Moreover, postural orthostatic tachycardia was observed in a healthy patient 6 days postvaccination with the first dose of the Pfizer vaccine [40]; this type of arrhythmia had been previously observed in recipients of the Gardasil vaccine for human papillomavirus [41]. One possible explanation is that the autoimmune reaction against adrenergic receptors in the cardiovascular system leads to the impairment of vasoconstriction, subsequently causing postural tachycardia [40, 42]. Mustafa et al. suggested that the reduction of vasopressor response to plasma angiotensin-II and baroreflex compromise led to impaired vasoconstrictive response and subsequent orthostatic tachycardia in the upright position [43]. It should be noted that COVID-19 infection increases the possibility of developing arrhythmia by 3.83-fold [34] and that the reports of arrhythmia are at the level of case reports and have not been proven;. therefore, it would appear that taking vaccines is justifiable.
Myocardial Infarction
There have been reports of myocardial infarction in persons postvaccination with the Pfizer, AstraZeneca, and Sinovac vaccines, with the gap between vaccination and the occurrence of myocardial infarction varying from 15 min to 2 days [44,45,46,47]. There are several possible mechanisms to explain why this could happen: (1) immune thrombotic thrombocytopenia [23]; (2) a series of allergic reactions which lead to occlusion of coronary arteries and, consequently, myocardial infarction (i.e. Kounis syndrome) [48]; and (3) high demand and low supply due to vaccination stress in weaker patients [45]. In a multi-national cohort study conducted by Li et al. on the incidence of vaccine-induced adverse effects, the risk of myocardial infarction after vaccination increased with age [49]. However, all studies were inconclusive in determining a relationship between vaccination and myocardial infarction; Barda et al. reported the risk ratio for developing myocardial infarction as being 1.07 for recipients of the Pfizer vaccine compared to 4.47 for people who were infected by SARS-CoV-2 [34]. Further longitudinal investigations are required to establish causation [49].
Hypertension
Some investigations have shown that COVID-19 vaccines may be associated with high blood pressure. In a case series of nine patients with stage III hypertension, eight were symptomatic (malaise, headache, tingling in the mouth and diaphoresis) and had increased blood pressure. All eight patients had received the Pfizer/BioNTech vaccine and the ninth had received the Moderna vaccine [50]. In another study, six participants, among 113 patients, showed an average rise in systolic or diastolic BP at home by ≥ 10 mmHg during the first 5 days after receiving the first dose of Pfizer/BioNTech vaccine, compared with the 5-day period immediately preceding vaccination [51].
Takotsubo Cardiomyopathy
There are two published reports of Takotsubo cardiomyopathy following vaccination with the Moderna [52] and AstraZeneca [53] vaccines. In both cases, other possible diagnoses were ruled out by further workups, such as angiography. Of note, no precipitating infectious, inflammatory, emotional, or physical factors were mentioned in these cases and both patients recovered after receiving treatment.
Conclusion
As described in this article, cardiovascular complications (i.e., thromboembolic events, myocarditis/pericarditis, Takotsubo cardiomyopathy, arrhythmias, and myocardial infarction) in individuals following COVID-19 vaccination have been reported recently; however, for most of the complications reports, the association between vaccination and the complications has not been proved. These events are infrequent and do not affect the global risk/benefit assessment of COVID-19 vaccines. It should be noted that a report of a cardiovascular event in an individual after receiving a vaccine does not imply causality. Further longitudinal studies with control groups are needed to portray a more obvious picture of the topic. Physicians must be alert and be familiar with typical and atypical clinical manifestations of these cardiovascular events in order to diagnose and manage possible cases as soon as possible and mitigate adverse events.
References
Shirani K, Sheikhbahaei E, Torkpour Z, et al. A narrative review of COVID-19: the new pandemic disease. Iran J Med Sci. 2020;45:233–49.
Hu J, Wang Y. The clinical characteristics and risk factors of severe COVID-19. Gerontology. 2021;67:255–66. https://doi.org/10.1159/000513400.
World Health Association. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/table. Accessed 18 Oct 2021.
World Health Association. COVID-19 vaccines advice. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice. Accessed 17 Oct 2021.
Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16.
Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–81.
Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15.
Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–7.
Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–71.
Shiravi AA, Saadatkish M, Abdollahi Z, Miar P, Khanahmad H, Zeinalian M. Vitamin D can be effective on the prevention of COVID-19 complications: A narrative review on molecular aspects. Int J Vitam Nutr Res. 2020. https://doi.org/10.1024/0300-9831/a000676.
Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9–14.
Forni G, Mantovani A, Forni G, et al. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28:626–39.
Angeli F, Zappa M, Reboldi G, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection: one year later. Eur J Intern Med. 2021;S0953–6205(21):00307–11. https://doi.org/10.1016/j.ejim.2021.09.007.
Sadoff J, Davis K, Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination—response from the manufacturer. N Engl J Med. 2021;384:1965–6.
Burn E, Li X, Kostka K, et al. Background rates of five thrombosis with thrombocytopenia syndromes of special interest for COVID-19 vaccine safety surveillance: incidence between 2017 and 2019 and patient profiles from 20.6 million people in six European countries. medRxiv. 2021. https://doi.org/10.1101/2021.05.12.21257083.
Gupta A, Sardar P, Cash ME, Milani RV, Lavie CJ. Covid-19 vaccine- induced thrombosis and thrombocytopenia-a commentary on an important and practical clinical dilemma. Prog Cardiovasc Dis. 2021;67:105–7. https://doi.org/10.1016/j.pcad.2021.05.001.
Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699.
Mahase E. AstraZeneca vaccine: Blood clots are “extremely rare” and benefits outweigh risks, regulators conclude. BMJ. 2021;373:n931.
Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. https://doi.org/10.1136/bmj.n1114.
von Hundelshausen P, Lorenz R, Siess W, Weber C. Vaccine-induced immune thrombotic thrombocytopenia (VITT): Targeting pathomechanisms with bruton tyrosine kinase inhibitors. Thromb Haemost. 2021;121(11):1395–9. https://doi.org/10.1055/a-1481-3039.
Mehta PR, Apap Mangion S, Benger M, Stanton BR, Czuprynska J, Arya R, Sztriha LK. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination—A report of two UK cases. Brain Behav Immun. 2021;95:514–7. https://doi.org/10.1016/j.bbi.2021.04.006.
Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–101. https://doi.org/10.1056/NEJMoa2104840.
Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–11. https://doi.org/10.1056/NEJMoa2105385.
U.S. Food and Drug Administration. Joint CDC and FDA statement on Johnson & Johnson COVID-19 vaccine. 2021 https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine
Cines DB, Bussel JB. SARS-CoV-2 Vaccine-induced immune Thrombotic Thrombocytopenia. N Engl J Med. 2021;384(23):2254–6. https://doi.org/10.1056/NEJMe2106315. Epub 2021 Apr 16. Erratum in: N Engl J Med. 2021 Jun 10;384(23):e92. PMID: 33861524; PMCID: PMC8063912.
Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16(8):2142–5. https://doi.org/10.1016/j.radcr.2021.05.033.
Bautista García J, Peña Ortega P, Bonilla Fernández JA, Cárdenes León A, Ramírez Burgos L, Caballero Dorta E. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol (Engl Ed). 2021;74(9):812–814. https://doi.org/10.1016/j.rec.2021.04.005.
Ammirati E, Cavalotti C, Milazzo A, et al. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int J Cardiol Hear Vasc. 2021;34:100774.
Watad A, De Marco G, Mahajna H, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines. 2021;9:1–23.
Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D, Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W, Muhsen K, Levi Y, Green MS, Keinan-Boker L, Alroy-Preis S. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2109730.
Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, Kornowski R. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2110737.
Montgomery J, Ryan M, Engler R, et al. myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6:1202–6. https://doi.org/10.1001/jamacardio.2021.2833.
Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–90. https://doi.org/10.1056/NEJMoa2110475.
Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210–12. https://doi.org/10.1001/jama.2021.13443.
U.S. Centers for Disease and Prevention. Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines Among Adolescents and Young Adults. Available from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html. Accessed 13 Oct 2021.
American Heart Association/American Stroke Association. COVID-19 vaccine benefits still outweigh risks, despite possible rare heart complications. Available from: https://newsroom.heart.org/news/covid-19-vaccine-benefits-still-outweigh-risks-despite-possible-rare-heart-complications. Accessed 13 Oct 2021.
Peretto G, Sala S, Rizzo S, et al. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol. 2020;75:1046–57.
Marco García MT, Torres Lana Á, Anta Agudo MB, Rufino Delgado MT. Tachycardia as an undescribed adverse effect to the Comirnaty© vaccine (BNT162b2 Pfizer-BioNTech Covid-19 vaccine): Description of 3 cases with a history of SARS-CoV-2 disease. Enferm Infecc Microbiol Clin (Engl Ed). 2021 Mar 18:S0213-005X(21)00074-4. English, Spanish. https://doi.org/10.1016/j.eimc.2021.03.008. Epub ahead of print. PMID: 33858709; PMCID: PMC7969861.
Reddy S, Reddy S, Arora M. A case of postural orthostatic tachycardia syndrome secondary to the messenger RNA COVID-19 vaccine. Cureus. 2021;13:e14837.
Arana J, Mba-Jonas A, Jankosky C, et al. Reports of postural orthostatic tachycardia syndrome after human papillomavirus vaccination in the vaccine adverse event reporting system. J Adolesc Heal. 2017;61:577–82.
Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3:e000755.
Mustafa HI, Raj SR, Diedrich A, et al. Altered systemic hemodynamic and baroreflex response to angiotensin II in postural tachycardia syndrome. Circ Arrhythmia Electrophysiol. 2012;5:173–80.
Chatterjee S, Ojha UK, Vardhan B, Tiwari A. Myocardial infarction after COVID-19 vaccination-casual or causal? Diabetes Metab Syndr. 2021;15(3):1055–1056. https://doi.org/10.1016/j.dsx.2021.04.006.
Boivin Z, Martin J. Untimely myocardial infarction or COVID-19 vaccine side effect. Cureus. 2021;13:e13651.
Tajstra M, Jaroszewicz J, Gąsior M. Acute coronary tree thrombosis after vaccination for COVID-19. JACC Cardiovasc Interv. 2021;14:e103–4.
Özdemir İH, Özlek B, Özen MB, Gündüz R, Bayturan Ö. Type 1 Kounis syndrome induced by inactivated SARS-COV-2 vaccine. J Emerg Med. 2021. https://doi.org/10.1016/j.jemermed.2021.04.018.
Kounis NG, Mazarakis A, Tsigkas G, Giannopoulos S, Goudevenos J. Kounis syndrome: a new twist on an old disease. Future Cardiol. 2011;7:805–24.
Li X, Ostropolets A, Makadia R, et al. Characterizing the incidence of adverse events of special interest for COVID-19 vaccines across eight countries: a multinational network cohort study. medRxiv. 2021:2021.03.25.21254315.
Meylan S, Livio F, Foerster M, et al. Stage III hypertension in patients after mRNA-based SARS-CoV-2 vaccination. Hypertension. 2021;77:e56–7. https://doi.org/10.1161/HYPERTENSIONAHA.121.17316.
Zappa M, Verdecchia P, Spanevello A, Visca D, Angeli F. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine. Eur J Intern Med. 2021;90:111–3. https://doi.org/10.1016/j.ejim.2021.06.013.
Jani C, Leavitt J, Al Omari O, et al. COVID-19 vaccine-associated takotsubo cardiomyopathy. Am J Ther. 2021;28:361–4.
Crane P, Wong C, Mehta N, Barlis P. Takotsubo (stress) cardiomyopathy after ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021;14: e246580. https://doi.org/10.1136/bcr-2021-246580.
Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398:1377–80. https://doi.org/10.1016/S0140-6736(21)02046-8.
Li X-N, Huang Y, Wang W, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case–control real-world study. Emerg Microbes Infect. 2021;10:1751–9. https://doi.org/10.1080/22221751.2021.1969291.
Kang M, Yi Y, Li Y, Sun L, Deng A, Hu T, Zhang J, Liu J, Cheng M, Xie S, Luo M, Jiang J, Jiang Y, Tang S, He J. Effectiveness of inactivated COVID-19 vaccines against COVID-19 Pneumonia and Severe Illness Caused by the B.1.617.2 (Delta) Variant: Evidence from an Outbreak in Guangdong, China. Available at SSRN: https://ssrn.com/abstract=3895639 or http://dx.doi.org/10.2139/ssrn.3895639.
Barchuk A, Cherkashin M, Bulina A, et al. Vaccine effectiveness against referral to hospital and severe lung injury associated with COVID-19: a population-based case-control study in St. Petersburg, Russia. medRxiv. 2021. https://doi.org/10.1101/2021.08.18.21262065.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design, material preparation, data collection, and analysis. All authors read and approved the final manuscript.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Disclosures
Amir Abbas Shiravi, Ali Ardekani, Erfan Sheikhbahaei and Kiyan Heshmat-Ghahdarijani have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Amir Abbas Shiravi and Ali Ardekani are to be considered co-first authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shiravi, A.A., Ardekani, A., Sheikhbahaei, E. et al. Cardiovascular Complications of SARS-CoV-2 Vaccines: An Overview. Cardiol Ther 11, 13–21 (2022). https://doi.org/10.1007/s40119-021-00248-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-021-00248-0